pH is one of those chemistry concepts that you start to learn even before you can properly speak. For example, my pre-school niece knows that lemon juice is sour (a characteristic of acids, that have a low pH).
If you are anything like me, you may struggle to remember your high school chemistry, which is important if you want to understand discussions about pH and skincare. A few years back, pH was a massively trending topic across skincare, but not so much now.
Why? Manufacturers are under no obligation to disclose the pH of products, and some (e.g.) cleansers can be quite detrimental to your skin barrier – the ones that are very alkaline (or have a high pH). But consumers are not walking around carrying pH strips measuring every skincare product that they use.
Why am I so interested in pH?
I have started my Organic Skincare diploma with Formula Botanica and one of the topics getting the better of me, is pH. I am required to test the pH of anything containing water and especially when I add (e.g.) preservatives that could cause the pH to fluctuate.
Today’s blog
This blog summarizes basic chemistry surrounding pH. It is straight from A’Level chemistry books (I am British) and the Khan Academy. Thank you to both.
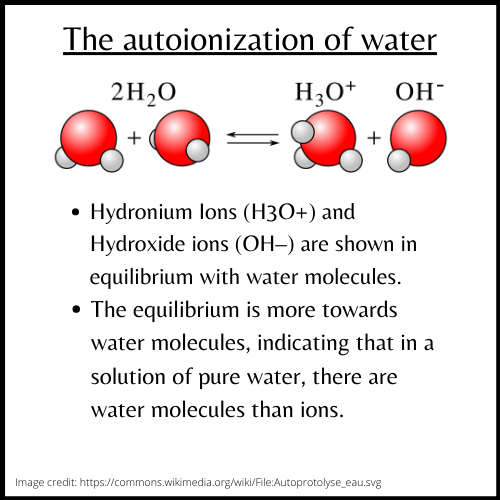
Water in equilibrium with its ions
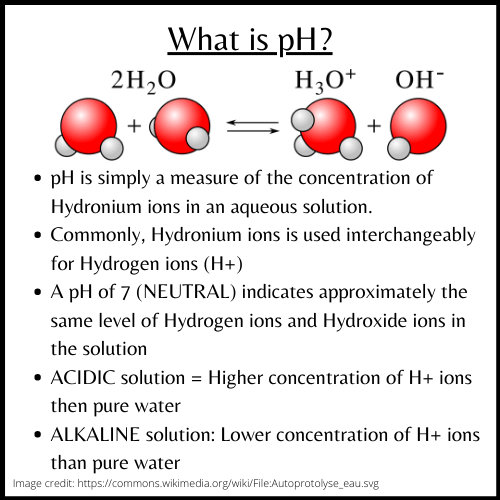
Textbook definition of pH
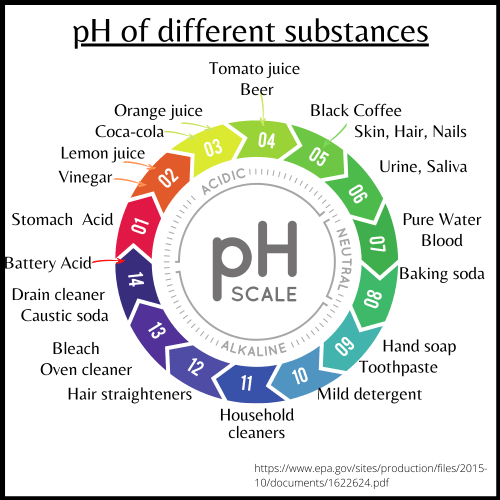
pH of substances that are measured by a reliable government agency
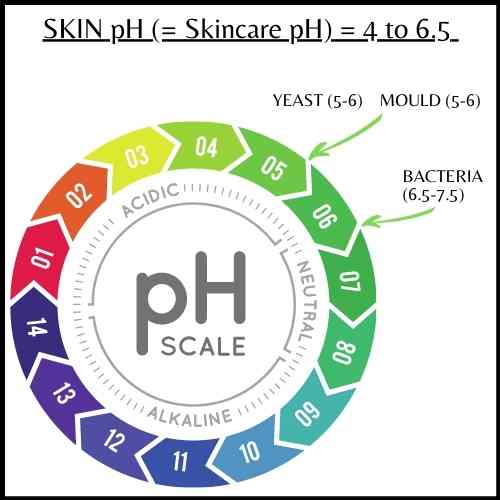
pH and microbial growth
One of the functions of preservatives and pH adjusters is to prevent microbial growth and spoilage of product. Why is pH important to microbial growth?
Different types of microbes have optimal pH ranges as follows. Remember that the skin’s pH is between 4 to 6.5 (its definitely more on the acidic side) and skincare products (are generally formulated) to respect this range.