My view on Sulisobenzone
Octinoxate, Octocrylene and Sulisobenzone have all been shown in dermal penetration studies to permeate the epidermis and the dermis. Secondly, insufficient data exists to establish the safety (or otherwise) record of Sulisobenzone. In over 90%+ sunscreens I have seen in the USA, India and Europe, I don’t encounter Sulisobenzone, simply because there are better substitutes. Would I use a sunscreen containing Sulisobenzone? Probably not because there are better alternatives available in the market.
Background
Sulisobenzone is also 2-hydroxy-4-methoxybenzophenone-5-sulfonic acid and its trade name is Uvinul MS 40.
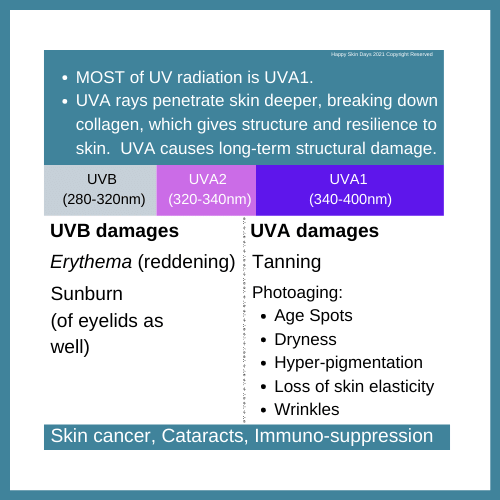
It is a weak broadspectrum UV filter that provides protection against both UVB and UVA radiation. (It is a better UVB filter than UVA filter). Recall that we want UV filters to protect against the following types of damage:
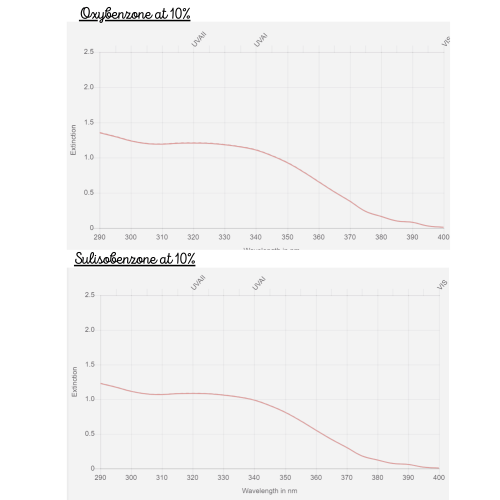
As you can see its absorbance is inferior to Oxybenzone at the same concentration.
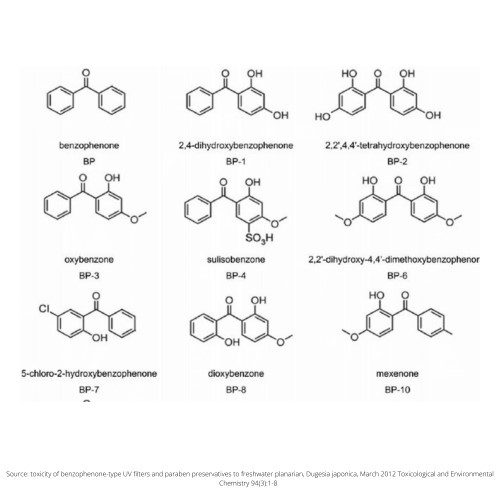
Structure
Clearly, Sulisobenzone belongs to the same family as Oxybenzone
Characteristics and safety
Unlike many UV filters in the market, Sulisobenzone is water-soluble AND not oil-soluble. These characteristic really limits its usefulness, because its then limited to daily-wear moisturizer type products. Even then, its not the first choice for manufacturers for 2 reasons. First, there are simply better alternatives in terms of protection against UV radiation available on the market.
Second, it “can be sticky in formulations and is not an optimum choice for daily moisturising products, particularly those used on the face, where more elegant formulations are necessary.”
Ensulizole (PBSA) is commonly substituted for Sulisobenzone.
Dermal penetration
It is clear that along with filters such as Octinoxate, Octocrylene etc, Sulisobenzone (or Benzophenone-4) does in dermal penetration studies, permeate the epidermis/dermis.
Unfortunately, there is no data or study that I can locate that talks to the side-effects (if any) of such percutaneous penetration.
What do the regulators say?
Sulisobenzone is permitted for use in the USA and Europe. (I haven’t checked Indian regulations yet). In the USA, the FDA (regulatory body) has said that along with most filters, there are data and/or information gaps on this filter. They want such gaps filled before they consider Sulisobenzone to be considered as GRASE.
Conclusion
This was a deeply strange blog to write, because Sulisobenzone really is a non-entity in the sphere of sunscreen filters and for this reason, no-one has done any extensive studies on it.
Sources and uses
sunscreen simulator
Kurul, E. and S. Hekimoglu, “Skin Permeation of Two Different Benzophenone Derivatives from Various Vehicles,” International Journal of Cosmetic Science, vol. 23(4), pp. 211-218, 2001.
Brinon, L., S. Geiger, V. Alard, et al., “Percutaneous Absorption of Sunscreens from Liquid Crystalline Phases,” Journal of Controlled Release, vol. 60(1), pp. 67-76, 1999.
21 CFR Parts 201, 310, 347 and 352 (Sunscreen Drug Products for Over-the-counter human use)
E. Kurul,S. Hekimoğlu, Skin permeation of two different benzophenone derivatives from various vehicles, International Journal of Cosmetic Science, Volume 23, Issue 4
He T et al Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral sp
Tangtian He et al, Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species, Science of The Total Environment, Volume 651, Part 2, 2019
Li M et al, Acute toxicity of benzophenone-type UV filters and paraben preservatives to freshwater planarian, Dugesia japonica, March 2012 Toxicological and Environmental Chemistry 94(3):1-8