This is part Deux of the Sunscren reboot.
I hope you are convinced that UVR is responsible for sunburn, skin cancer and photoaging and secondly, sunscreen is absolutely vital to preventing them.
If you are half as serious about your skincare routine as I am, then you need to identify the right sunscreen and make it part of your daily routine.
You still haven’t told me why I should read the label?
You should look for adequate quantities of UV filters. Sunscreens contain ingredients called ‘UV-filters’ that do the heavy lifting and determine how effective the product will be. Filters are substances protect the skin against certain UVR by absorbing, reflecting or scattering UV radiation.
Not all UV filters are born equal and second, some sunscreens are better than others AND COUNTRY REGULATION has a big hand in determining how effective your sunscreen is.
The simple reason to read the label is to align what you think you are buying with what you are actually buying.
What does a sunscreen with the best filters look like?
An ideal sunscreen has photostable UV filters, which don’t breakdown at the first sign of sunlight and absorb 100% of UVB and UVA.
The absorbance profile of the sunscreen would be like this.
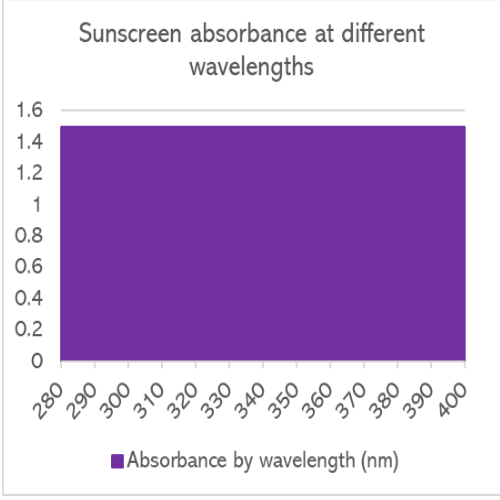
No such sunscreen exists. This is a complete fiction.
1) SUNSCREEN REGULATION
I buy sunscreen in 3 territories – the US, the EU and India. All three could not be more different…
1A) Indian regulation of sunscreen
India is easy as pie. Neither skin cancer nor photo-aging are public health issues, and sunscreen regulation is absolutely minimal. Sunscreens are regulated as cosmetics and they can only use filters – UVA and UVB – from a prescribed list that mirrors the EU list.
I don’t anticipate this will change: its hard to make the case that photoaging should be viewed as a public health issue.
What does it mean for consumers?
Manufacturer’s have a pretty free hand on what filters and claims they put on packaging. There is no minimum SPF, no prescribed labelling and significantly, there is no requirement for sunscreens to contain filters that act in both the UVA and UVB region.
For example, Shahnaz Husain uses the word “sunblock,” Kaya puts “sweat proof” and VLCC uses SPF 100+. None of these manufacturers have had to pass a broadspectrum or water resistant test, which they would have had to do in the EU or the USA.
1B) US regulation of sunscreen
Before I outline the current rules on American sunscreen, you need to know that the American regulator – Food & Drug Administration – published draft proposals earlier this year, that may change a lot of what I have written.
Under current rules, ALL products that contain one or more permitted UV filters are treated as SUNSCREENS. Sunscreens are (mainly) regulated as OTC sunscreen drugs.
It is completely optional for sunscreens to provide UVA and UVB absorbance. UVA and UVB absorbance is referred to as “Broad Spectrum” on American sunscreens, but here is the thing – this is not mandatory for sunscreens.
Therefore, you can buy a “sunscreen product that does not pass the broad spectrum test,” in which case it will have a warning (eg) “Skin Cancer/Skin Aging Alert; Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.”
Broadspectrum sunscreens can carry this label: if used as directed with other sun protection measures (see Directions, decreases the risk of skin cancer and early skin aging caused by the sun”.
The “Broad Spectrum” test
Sunscreens can only carry the words “broad spectrum” if they have passed this test: the absorbance of the sunscreens is tested at 1nm wavelengths between 290nm and 400nm.
The area under the curve represents absorbance of UVA and UVB: 90% of this area between 290 nm and 400nm has to occur at 370nm (called the critical wavelength). This is a pass or fail test.
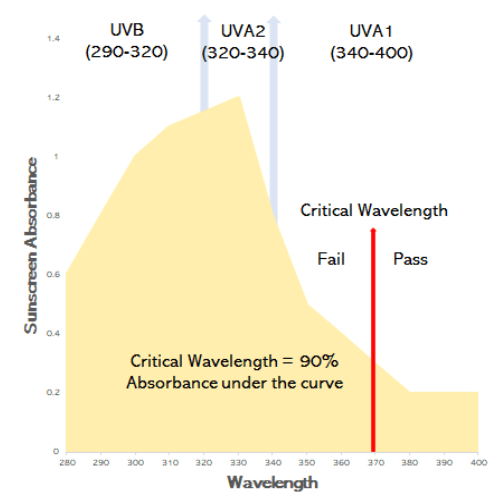
Because the absorbance of the sunscreen occurs over UVB (290-320nm) and UVA (320-400nm), its called “broadspectrum.”
The problem with the critical wavelength test
This is straight from the horses mouth: the US FDA says, “
we are concerned about the existing potential for inadequate UVA protection in marketed sunscreen products.
The FDA explains that HIGH SPF products may not provide adequate UVA protection. Its still possible for both product 1 and product 2 in the diagram below to pass the critical wavelength test.
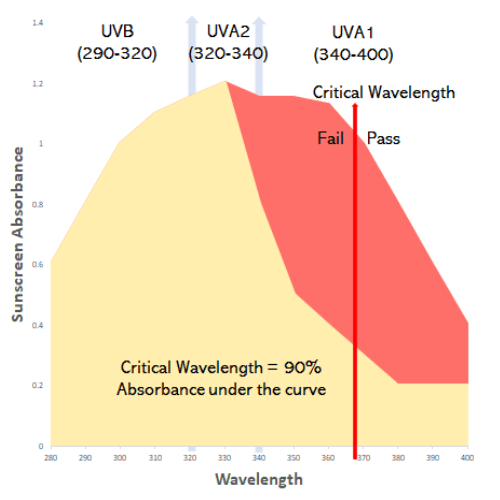
1C) So, what’s the big deal if SPF 100+ has less UVA protection than SPF30?
The International Agency for Research on Cancer says that high SPF sunscreen products are associated with longer intentional UV exposure.
Basically, everyone wearing SPF 100+ thinks that their skin is bulletproof and that UV exposure is never going to affect them.
Sun damage is cumulative over the years and a lack of UVA filters in Indian and to a lesser extent, American sunscreens, means that while you think you are protecting your skin against photoaging, immunosupression and skin cancer – you are not.
1D) EU regulation of sunscreens
EU regulation is by far not perfect, but it plugs in the gaps in UVA filter that are presented by American and Indian regulation.
There are specific rules that Made and Sold in the EU products must comply. These are:
(i) Sunscreens must have a minimum SPF of 6
(ii) In all sunscreens, the total UVA protection as a fraction of SPF, must be 1/3
(iii) All sunscreens must pass the critical wavelength test of 370nm (see bove)
These rules ensure that:
- UVA absorbance is bolstered by UVA/Total SPF being one-third of SPF.
- As the SPF of the product increases, so does the UVA absorbance of the product.
Therefore, an SPF 30 and an SPF 50 product will both be required to have UVA absorbance = 1/3 of SPF.
1E) Do American sunscreens meet the more stringent requirements of EU for UVA absorbance?
The only way to actually tell this is testing American sunscreens in a lab. I found one limited study, that looked at the UVA absorbance of 20 bestselling sunscreens. All provided UVA protection via the critical wavelength test, but more than half of these products failed the EU test.
Thankfully, the FDA has a taken a position as it says
We are …proposing (that) to pass the broad spectrum test, a product must demonstrate that it provides a UVA I/UV ratio of 0.7 or higher…(and meet)… the 370 nm critical wavelength requirement.
The FDA continues to say that this eliminates the possibility of
a sunscreen that is labeled “broad spectrum SPF 30” could provide less UVA protection than a sunscreen labeled “broad spectrum SPF 15.”
Other blogs you may be interested in are:
How to buy sunscreen, Myths about sun-protection
Sources and uses
UV-A PROTECTION AND SUNSCREENS CIE Technical Report ISBN 978 3 901 906 80 0
COMMISSION RECOMMENDATION of 22 September 2006 on the efficacy of sunscreen products and the claims made relating thereto (notified under document number C(2006) 4089) (Text with EEA relevance) (2006/647/EC)
Guidelines to Commission Regulation (EU) No 655/2013 laying down common criteria for the justification of claims used in relation to cosmetic products Ref. Ares(2015)4340493 – 16/10/2015
Frank Padera, PerkinElmer, Inc. Shelton, CT Sunscreen Testing According to COLIPA 2011/FDA Final Rule 2011 Using UV/Vis LAMBDA Spectrophotometers
Steven Q. Wang • Henry W. Lim Editors, Principles and Practice of Photoprotection ISBN 978-3-319-29381-3
Sunscreen Drug Products for Over-the-Counter Human Use, A Proposed Rule by the Food and Drug Administration on 02/26/2019
Skin Cancer Facts & Statistics https://www.skincancer.org/skin-cancer-information/skin-cancer-facts/
The Austrian UVA-Network. Photochem Photobiol. 2019 Apr 24. doi: 10.1111/php.13111 https://www.ncbi.nlm.nih.gov/pubmed/31017671
Annex VI, Last update: 05/08/2019, LIST OF UV FILTERS ALLOWED IN COSMETIC PRODUCTS
https://ec.europa.eu/growth/tools-databases/cosing/pdf/COSING_Annex%20VI_v2.pdf
Krutmann J, Bouloc A et al, The skin aging exposome Journal of Dermatological Science 85 (2017) 152–161 http://dx.doi.org/10.1016/j.jdermsci.2016.09.015
Autier, P., M. Boniol, and J.F. Dore, “Sunscreen Use and Increased Duration of Intentional Sun Exposure: Still a Burning Issue,” International Journal of Cancer, vol. 121(1), pp. 1-5, 2007.
