Occasionally, I’ll write a special interest blog, which is not necessarily on top of everyone’s agenda but is of import.
This is such a blog.
Every month, there is (supposedly) a new clinical trial / finding (mainly emerging from the US or Australia) that puts the spotlight on sunscreen use. The internet and social media burst into action and everyone and their dog is trying to answer this question:
Do the benefits of using sunscreen outweigh its disadvantages?”
For example, fresh off the Australian press this NOTE.
Where do I fit in?
I want to steer you away from the chaos and persuade you that as consumers, the question is not, “Should I be using sunscreen?” but, “What is my country regulator doing to ensure my sunscreen is as safe and affordable as possible?”
Before that context as…
Debate without context is the death of reason
First, this currently only affects “Made in America” sunscreens because they are based on archaic rules that permit only old-school filters. Major American sunscreen regulation was last introduced in 1999, based on available evidence at that time. We didn’t know about the serious damaged caused by UVA at that time…
The 1999 rules permitted sunscreens (an OTC drug in the USA) to use only 16 filters.

In addition, photounstable Avobenzone could not be combined with Zinc Oxide and Titanium Dioxide.
In my view*, this has bifurcated the American sunscreen market as follows:
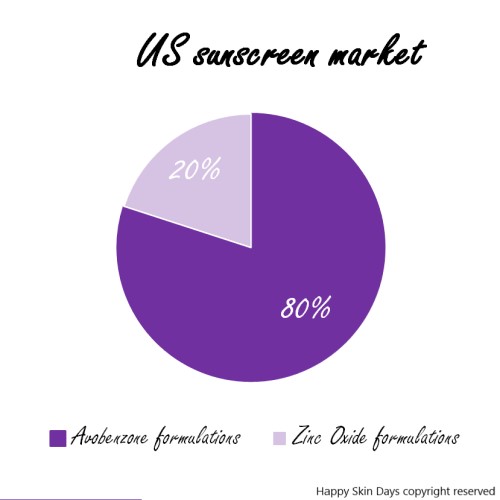
*Based on my review, the most common filters are: OCTOCRYLENE, AVOBENZONE, OCTISALATE, HOMOSALATE, OXYBENZONE, ZINC OXIDE AND TITANIUM DIOXIDE

Seven out of 16 filters, does not leave a lot of space for manufacturer’s to create products that are supremely different from each other….And they don’t: each of these products contains 21.6% Zinc Oxide and has a similar formulation:
The products are:

(L to R: Neutrogena SheerZinc Dry-Touch SPF 50 (Most expensive), Aveeno Positively Mineral Face sunscreen SPF 50 (34% cheaper), CVS Health Zinc Face Sheer Lotion SPF 50 (61% cheaper), CVS Health Zinc Sheer Lotion SPF 50 (68% cheaper))
What is missing from the current debate ( internet and social media coverage) that you need to know?
Its the FDA’s monograph on sunscreens published in February 2019. This is the LINK.
In the document, the FDA acknowledges that its been 20 years since they looked at the safety of current UV filters. The FDA says that it needs additional safety data to establish that some sunscreen filters are still GRASE.
This document is also a comprehensive review of the American sunscreen landscape, looking at basically everything (clinical trials, studies etc) on each individual sunscreen filter and outlines what additional data the FDA is looking for. Using Avobenzone as an example, the FDA monograph says the data gaps are:
- Pharmacological studies – Human absorption of Avobenzone(including metabolite study in humans)
- Nonclinical safety studies (toxicokinetics, dermal carcinogenicity, systemic carcinogencity of Avobenzone)
And other studies (e.g.) pediatric studies may be necessary depending on above outcome.
This seems like a perfectly reasonable approach for any regulator to take.
Which of the 16 filters are safe for use in sunscreens?
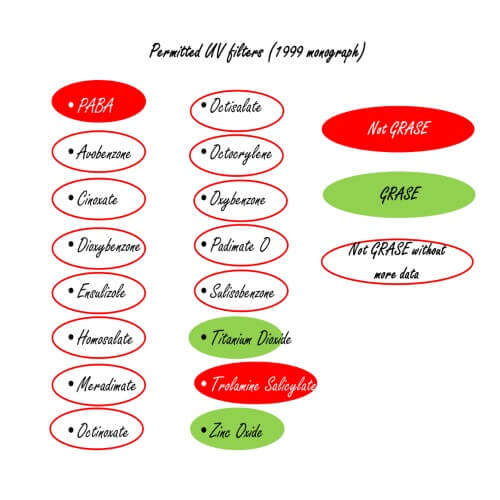
The FDA have said:
- ONLY Titanium Dioxide and Zinc Oxide are GRASE – no further data is necessary for these UV filters.
- PABA and Trolamine Salicylate are not GRASE – they have enough data for this.
All other UV filters are NOT GRASE until the FDA is able to obtain additional data. It asks manufacturers for such data. The monograph does not recommend that manufacturers withdraw said ingredients from sunscreens.
This monograph basically puts manufacturers on notice – cough up the data OR run more trials, otherwise there is a real prospect that many UV filters won’t be GRASE.
It is unsurprising that the FDA then received over 20,000 comments.
What else?
The most recent clinical trial sponsored by the FDA started in Jan/Feb 2019 – coincidentally around the time the monograph was published. While the FDA has asked interested parties (including pharma companies) to produce data asking to support GRASE status, at the same time its completely building its own body of evidence – which is what the study is about. That is what you kind of would expect of a regulator.
It appears that the next time we hear from the FDA on the status of the UV filters is September 2020 after the FDA goes through its 20,000+ comments.
How affected are we or the Americans by these changes?
There is no uniform “sunscreen code” that governs sunscreens sold in (e.g.) the EU, Japan, Australia or India. Each country decides what UV filters will be permitted or not.
The EU permits 31 filter.
I do not live in the USA and quite frankly, I can avoid old school UV filters and opt for newer filters (e.g.) BEMT that are larger in size and won’t be absorbed by my skin.
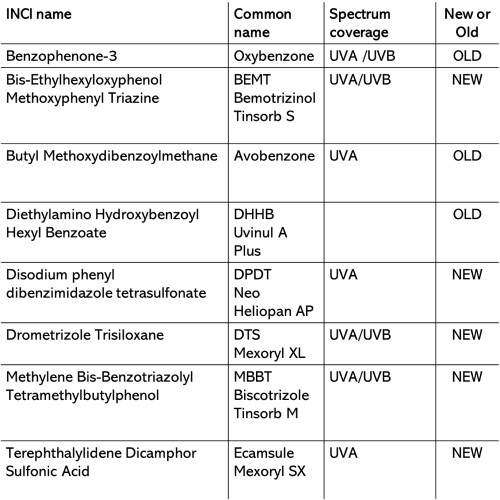
We do not need to suffer in the EU…
Avobenzone is extremely commonly used in EU sunscreens too BUT there are a small number of both mineral sunscreens and non-Avobenzone based sunscreens.
Here are a few that are on my radar:
(L to R Noreva Cicadiane Protect Photoprotective Repairing Care SPF 50+, Ultrasun Face Brightening Anti-Spot & Anti-Pollution Fluid SPF 50+, Ultrasun FACE ANTI-PIGMENTATION SPF50+, Ultrasun Face Very High SPF50+ Anti-Ageing Tinted in Ivory, SVR CICAVIT + crème SPF50 +, Anglamark Sun Face SPF 50)
What options do American consumers have?
Option 1: continue to use existing sunscreen
The FDA has said there is no reason currently for manufacturers to change their sunscreen formulations. Just because something enters your blood stream doesn’t mean its harmful or that your body can’t process it. Also, if the levels of a UV filter in your blood are higher than expected, then this does not (again) mean that your body is unable to cope OR that you should throw out your sunscreen.
Option 2: switch to mineral sunscreens
A mineral sunscreen has either or both Zinc Oxide and Titanium Dioxide.
Mineral sunscreens have a very long safety record. This BLOG tells you everything you need to know about Titanium Dioxide and Zinc Oxide.
This blog gives you Zinc Oxide only formulation options that you can buy in CVS pharmacy.
Option 3: don’t use sunscreen
Whatever you do, don’t do this. This debate is not about whether or not you should stop using sunscreen – its about the regulator (the FDA) forcing through change in a billion dollar plus industry that has not changed for the past 20 years.
P.S. Every single medical and health body supports the use of sunscreen. The American Cancer Society[1] says “most skin cancer cases and deaths are caused by exposure to UVR and thus potentially preventable.” Sunscreen with SPF 30 is recommended as one of the ways to minimize UVR exposure.
Skin cancer affects over 3 million people in the USA alone and is the most common type of cancer.
If you live in the USA and are unsettled by the current sunscreen debate, please use a mineral sunscreen.
In closing
I hope I have convinced you that the current debate is a tug-of-war between the FDA and sunscreen manufacturers, who have a lot at stake.
The worst thing that could happen if you are a manufacturer is the US FDA bans Avobenzone. That leaves ONLY Zinc Oxide as the primary UVA filter and trust me there are not 20 ways you can formulate a 21.6% Zinc Oxide sunscreen and justify charging 100 times more than your closest competitor.
NOTHING could also happen. The will of the FDA is being tested by the current political climate in the USA and quite frankly, if I was a betting man I’d put my money on no change.
However, the question for consumer is (i) what is my regulator doing to ensure that my sunscreen is safe as possible AND (ii) why is sunscreen not more affordable?
P.S. What clinical trial?
Recently, an American clinical trial tested for the systemic absorption of 6 active sunscreen filters that were present in 4 regular sunscreens products under single use conditions (one application on day one) and maximum use conditions (4 applications on other days).
The published results fund ALL 6 sunscreen ingredients tested resulted in exposure over 0.5 mg/mL. This threshold was breached on day one (singe use conditions).
The 6 sunscreen ingredients are Avobenzone, Oxybenzone, Octocrylene, Homosalate, Octinoxate, Octisalate.
The thing is, is anyone brutally surprised by this? We’ve known that Oxybenzone has been found in the urine sample of over 90% of Americans….this is old news. Is it that surprising that chemical filters are found in our bloodstream when our skin is “permeable?”
Sources and uses
https://www.ncbi.nlm.nih.gov/pubmed/24945639
https://www.govinfo.gov/content/pkg/CFR-2011-title21-vol5/xml/CFR-2011-title21-vol5-part352.xml
Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients, A Randomized Clinical Trial JAMA. 2020;323(3):256-267. doi:10.1001/jama.2019.20747
https://www.tga.gov.au/book/export/html/5307
https://clinicaltrials.gov/ct2/show/NCT03582215
https://www.tga.gov.au/publication/australian-regulatory-guidelines-sunscreens-args
https://www.legislation.gov.au/series/F2019L00834
https://www.ncbi.nlm.nih.gov/pubmed/24945639
[1] https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html
Photo by Pineapple Supply Co. from Pexels
