(All 50 references to the Acid series are at this LINK)
If you are an expert or have a PhD on organic chemistry please don’t read my blog to pick holes in my high school chemistry. Don’t be that person – its not cool .
The purposed of today’s blog
I’ll be giving you a basic chemistry lesson, which I hope will demystify the (simple) world of exfoliating acids with more confidence, whether its on my blog or elsewhere!
What is a functional group?
Literally, the textbook definition is: “an atom or group of atoms in an organic molecule that determines the characteristic reactions of a homologous series.”
For example, the functional group for alcohol is – OH or the Hydroxyl group.
When -OH is attached to a single Carbon atom, this forms the simplest alcohol, methanol.
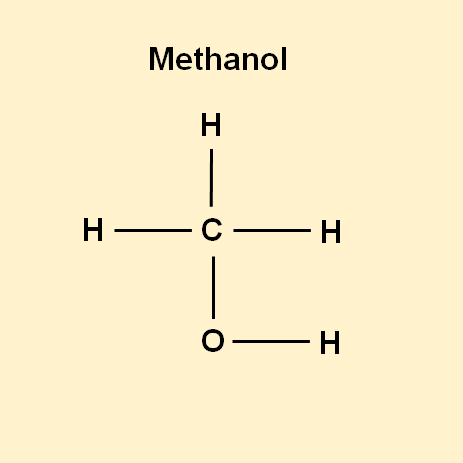
The -OH group dictates the reactions of methanol. For example, methanol will easily dissolve in water, because of the -OH group’s affinity for water.
Similarly, methanol has a higher boiling point for such a small molecule because of the -OH group that facilitates for stronger (Hydrogen) bonding between different molecules of methanol.
Organic ingredients have complicated structures (e.g.) they can have multiple functional groups present in the same molecule. These groups along with other factors determine how the molecule reacts.
That’s it…
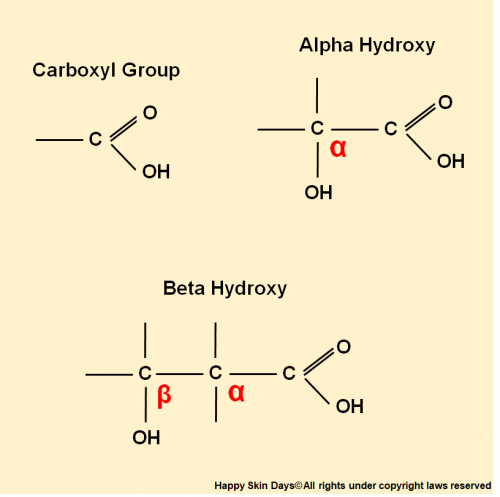
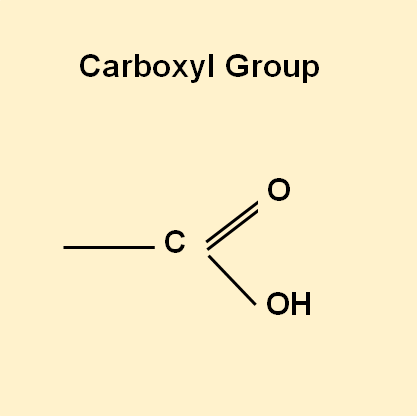
Carboxylic Acids – the functional group of exfoliating acids
Carboxylic acids are weak acids that have this Carboxyl group in common
Structures of the most common carboxylic acids used in skincare
These are Glycolic Acid, Salicylic Acid and Lactic Acid.
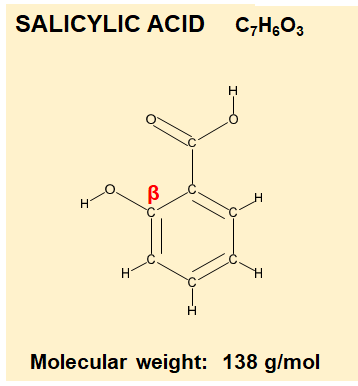
The “ring” in Salicylic Acid essentially makes this molecule lipophilic or fat loving. These means its soluble in fat, unlike Glycolic Acid or Lactic Acid.
There are fewer incidences of other acids – you simply cannot buy them unless you are looking for them. These acids are individually or collectively less common ingredients and I’ve included them because I am a geek.
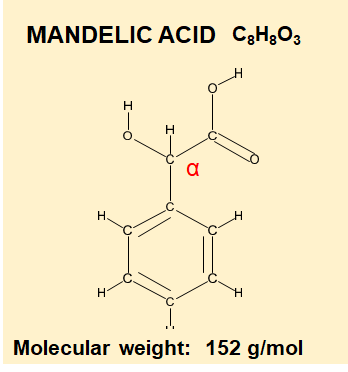
Mandelic Acid is an Alpha Hydroxy Acid.
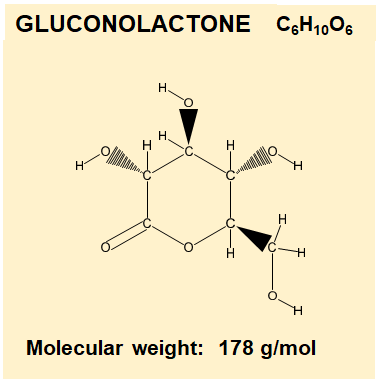
Gluconolactone is a Poly Hydroxy Acid
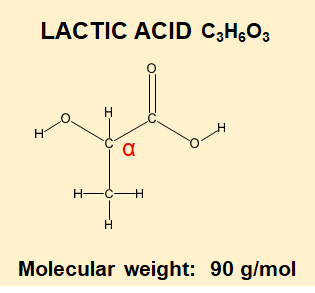
How do Beta and Alpha Hydroxy Acids get their name?
Alpha, Beta are letters of the Greek alphabet and are used to help locate functional groups in a molecule.
When you see AHA or BHA then this refers to Alpha Hydroxy- and Beta Hydroxy – and all that means is the location of the -OH group changes from one Carbon atom to another.