Its that time of the year when I am going to do a sunscreen-blitz and review 50+ sunscreens (because only the good stuff is launched in the summer months).
I love reviewing Indian sunscreen’s because the country has no separate sunscreen regulation and, there is such creativity in marketing claims.
For example, the product that will not be named, is called “Guneera Gel” (sorry I think I just named it) and advertises common sunscreen filters on the label.
There is no claim to this being a sunscreen or having an SPF. In fact (I kid you not), SPF 50 is split into sections, so if you are not looking carefully, you won’t notice 50 appears much below the word SPF, so that you could easily be mistaken that “50” and “SPF” are not meant to appear together.
Percentages of sunscreen filters are profusely displayed BUT not the entire ingredient list. The filters are the usual old-school suspects: Octinoxate, Avobenzone, Oxybenzone, Octocrylene. Nothing to get excited about (or literally get out of bed for).
I hold a high ethical standard when it comes to cosmetic formulations and I am a by the book type of person: so when I review products for my blog, its my earnest wish and desire that labels comply with labelling guidelines dictated by the law of the land.
The mere fact that this product did not name itself as a sunscreen, and has “base” for an ingredient list, outraged me. To reflect my outrage, I had already written a vitrolic and contemptuous introduction to my blog.
However, I decided to recheck the rules relating to cosmetic labelling and it turns out, that this 60ml product has labelling that is 100% compliant with Chapter VI of the Cosmetic Rules 2020.
Yes, please read that again.
I won’t bore you with the details today, but in the land of the small SKU unit (ie India), there are going to be an enormous number of products that fall within multiple exemptions (see below) and disclose the minimum amount to consumers.
There are no laws that prevent small indie brands and billion dollar brands from taking advantage of these rules.
Clearly I am in shock (I am easily stunned and surprised too by the way), so I will be writing this issue further…
What is a cosmetic?
Therefore, this blog is part of a short series revisiting the main points about the labelling of cosmetics, that are Made in India (that is, manufactured, packaged and labelled in India)
But first, what is a cosmetic?
Pursuant to Section 1(3)of the Drugs and Cosmetics Act, 1940,
“cosmetic” means any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance, and includes any article intended for use as a component of cosmetic
This pretty much includes all sunscreens.
What rules regulate labelling of cosmetics and what do they say?
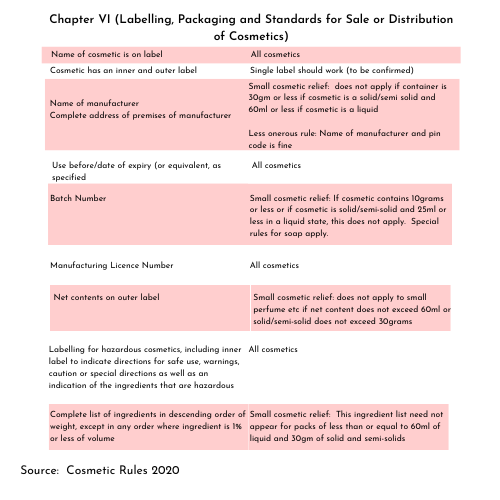
The Cosmetic Rules 2020[1] (yes quite recent) outline the requirements for labelling as follows under Chapter VI (Labelling, Packaging and Standards for Sale or Distribution of Cosmetics).
What are the main requirements for cosmetic labelling?
In a nutshell, the cosmetic must have:
- An inner label and an outer label
- For both the inner and outer label:
- Name of cosmetic
- Name of manufacturer
- Complete address of the premises at which the cosmetic is manufactured
- Use before or date of expiry (variants of this
- Batch number
- Manufacturing Licence number
- For the outer label
- Net content
- For cases where a hazard exists, the inner label shall indicate:
- Directions
- Warning
- Statement of use
- If there is only one label, the single label has to contain everything
- A complete list of all ingredients (with specifications below one per cent)
- The cosmetic needs to comply with labelling requirements (if relevant) as laid down by the Bureau of Indian Standards
What are the exemptions to this?
India is the land of the small SKU and essentially (and I kid you not), these very detailed requirements do not apply as follows and this is best represented in a pictorial format (yes, this is a picture for me)